Note 2: The use of 'nor' in norbornane to indicate the loss of three methyl groups is common but is discouraged. This usage should not be applied to any other monoterpenoid systems.
RF-4.1. Removal of Skeletal Atoms without affecting the Number of Rings
RF-4.1.1. The removal of an unsubstituted skeletal atom, saturated or unsaturated, from a ring or an unsubstituted skeletal atom from an acyclic portion of a fundamental parent structure with its attached hydrogen atom(s) is described by the prefix "nor-"; the loss of two or more skeletal atoms is indicated by combining an appropriate numerical prefix with "nor-", e.g. "dinor-", "trinor-", etc.
Note 1: The provisional Section F Rules (ref 1, 2) require the skeletal atoms removed to be saturated carbon atoms by using the prefix "nor" to indicate the removal of methylene groups. The carotenoid recommendations 5.1 (ref 5) provide that "nor" be used to indicate the removal of CH groups as well. These revised recommendations are more precise by permitting removal of CH groups only in a ring having the maximum number of noncumulative double bonds; they are also more general by allowing "nor" to indicate the removal of heteroatoms.The position of the skeletal atom that is removed is denoted in all cases by its locant in the numbering of the parent structure.Note 2: The use of 'nor' in norbornane to indicate the loss of three methyl groups is common but is discouraged. This usage should not be applied to any other monoterpenoid systems.

Note: A capital letter, associated with the locant of a skeletal atom where needed, has been used with prefixes such as "nor-" and "dinor-" to indicate removal of methylene groups from a particular ring. This system was recommended in the 2nd edition of the steroid rules [2S-7.1 (ref 9)] and is still used in Chemical Abstracts index nomenclature, but is not included in Section F because it is not as general as the locant system recommended here.Although, because the locant of each skeletal atom removed is cited, an unambiguous name can be generated by the removal of any skeletal atom, it is traditional to remove skeletal atoms with the highest possible locant in an atomic connector in a cyclic portion of the skeletal structure. An atomic connector is a chain of homogeneous skeletal atoms of the same element connecting any combination of bridgehead or ring junction atoms, rings or ring systems (i.e., ring assemblies), substituted skeletal atoms in the parent structure, or heteroatoms. In an acyclic portion of a skeletal structure, the skeletal atom removed preferably is the one of an acyclic atomic connector or a terminal segment nearest to the free end of the acyclic part of the structure. (This is done in order to maintain as far as possible traditional numbering of structural features of the compound and of compounds derived from it.) A terminal segment of a skeletal structure is an acyclic segment of homogeneous skeletal atoms connected at only one end by the features of structure that terminate atomic connectors (see above).
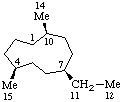 | 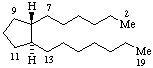 |
| 13-Norgermacrane | 1,20-Dinorprostane |
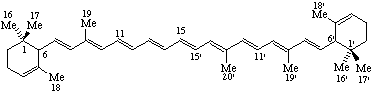
20-Nor-ε,ε-carotene
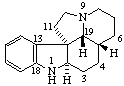 | 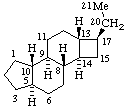 |
| 20,21-Dinoraspidospermidine | 4,16,18,19-Tetranor-5α-pregnane (has been called A,D(15),18,19-Tetranor-5α-pregnane) |
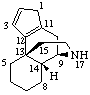
RF-4.1.2 When the removal of an unsaturated skeletal atom from a ring containing the maximum number of noncumulative double bonds in the fundamental parent structure results in the creation of a saturated ring position, this position is described by indicated hydrogen symbolism.
Example:
1. International Union of Pure and Applied Chemistry, "Nomenclature of Organic Chemistry: Section F - Natural Products and Related Compounds, Recommendations 1976", IUPAC Information Bulletin Appendices on Tentative Nomenclature, Symbols, Units, and Standards, No. 53, December, 1976. [also in: Eur. J. Biochem. 86, 1-8 (1978)].
2. International Union of Pure and Applied Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, l979 edition, Pergamon Press, Oxford, 1979.
4. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Joint Commission on Biochemical Nomenclature, "Nomenclature of Steroids", Pure Appl. Chem., 61, 1783-1822 (1989). [also in: Eur. J. Biochem., 186, 429-458 (1989) and pages xxx-lix in Dictionary of Steroids (Hill, R.A., Kirk, D.N., Makin, H.L.J. & Murphy, G.M., eds) Chapman & Hall, London 1991].
5. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Commission on Biochemical Nomenclature, "Nomenclature of Carotenoids", Pure Appl. Chem., 41, 405-431 (1975).
9. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Commission on Biochemical Nomenclature, "The Nomenclature of Steroids Revised tentative rules, 1967.", Pure Appl. Chem., 31, 285-322 (1972). This 2nd edition has been superceded by the 1989 edition (ref 4).