Contents of this section
RF-10.1. Fundamental and Modified Parent Structures
Names of the fundamental and modified parent structures (see RF-3 through RF-7) imply, without further specification, absolute configuration at all chiral elements as depicted in these recommendations and the following Appendix.
When a planar or quasi-planar system of rings is denoted as a projection on paper, as in these recommendations, an atom or group attached to the ring is called α if it lies below or β if it lies above the plane of the paper. Use of this system requires the orientations of structure as given herein. In the example below, the implied configuration shown defines the attached hydrogen atoms or methyl groups at positions 8, 10, and 13, as β-, and at positions 9 and 14 as α-; here, the hydrogen atom at the chiral position 5 is not known and thus the orientation is ξ (xi). In the case of a racemate, the enantiomeric structure drawn should be the one that shows the lowest numbered chiral center in the α configuration (see also RF-10.5). This may differ from the usual practice, which is to draw the enantiomeric structure having the same absolute configuration as the naturally occurring substance.
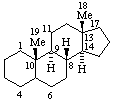
RF-10.2. Stereochemical Configurations that are Different
Stereochemical configurations that are different from those in the parent structure or that have been generated by substitution, etc.
RF-10.2.1. At chiral centers, the α/β system as described above or by 3S-1.4 in the IUPAC-IUB recommendations for the nomenclature of steroids (ref 4) and Rule E-4.11 of the IUPAC Nomenclature of Organic Chemistry (ref 2) is used [see also Section 2 in the recommendations for the nomenclature of Vitamin D (ref 6)]. A change in configuration of a non-bridgehead side-chain that is part of the parent is denoted by the method specified for C-17 of steroids [see 3S-5.2 (ref 4)], where the α or β refers to the side-chain itself and not to the hydrogen atom at the same position.
Examples:
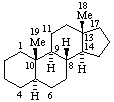 | 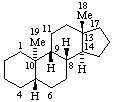 |
| 5α-Androstane (fundamental parent structure) | 5β,9β,10α-Androstane |
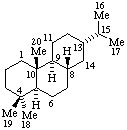 | 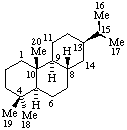 |
| Abietane (fundamental parent structure) | 13β-Abietane |
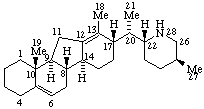
Veratraman (fundamental parent structure)
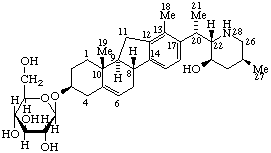
(23R)-23-Hydroxy-14,15,16,17-tetradehydroveratraman-3β-yl β-D-glucopyranoside
RF-10.2.2. When the α/β method is not applicable or is not acceptable for the specific natural product class, the R/S symbolism of the Sequence Rule System is used.
Examples:
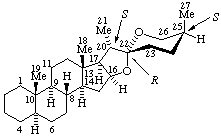 | 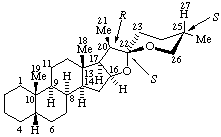 |
| (25S)-5α-Spirostan (fundamental parent structure; configurations at positions 5 and 25 are not implied) | (22S,25S)-5β,8α,16β,20β-Spirostan |
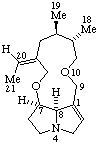 | 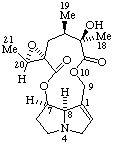 |
| Senecionan (fundamental parent structure) | (20R)-12-Hydroxy-15α,20-epoxysenecionan-11,16-dione |
RF-10.2.3. The descriptors cis/trans or E/Z are used to describe modified or additional stereochemical configurations for double bonds.
Example:
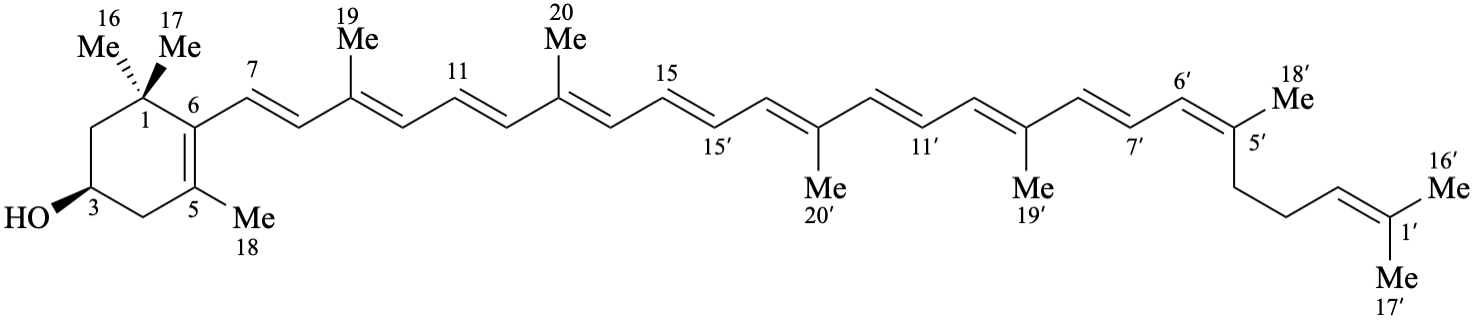
RF-10.3. Configurational Inversion at All Asymmetric Centers
Configurational inversion at all chirality centers is indicated by the italicized prefix ent- (a contracted form of enantio-) placed in front of the complete name of the compound. This prefix denotes inversion at all chirality centres (including those due to named substituents) whether these are cited separately or are implied in the name.
Examples:
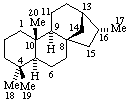 | 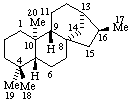 |
| Kaurane (fundamental parent structure) | ent-Kaurane |
Note: There is confusion in the literature here; some authors (and Chemical Abstracts) use Kaurane for the enantiomer called ent-Kaurane above.
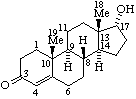 | 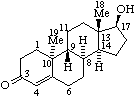 |
| 17α-Hydroxyandrost-4-en-3-one | ent-17α-Hydroxy-13α,14β-androst-4-en-3-one or 17β-Hydroxy-8α,9β,10α-androst-4-en-3-one (see RF-10.2.1) (but not 17β-Hydroxy-ent-13α,14β-androst-4-en-3-one) |
RF-10.4. Configurational Inversion at One Asymmetric Center
Configurational inversion at one asymmetric center whose configuration is implied or stated in the name for the fundamental parent structure can be indicated by the italicized prefix epi- (derived from word "epimer") placed in front of the name of the parent structure and prefixed by the locant of the affected atom.
Examples:
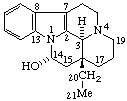 | 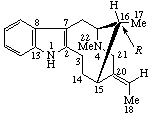 |
| 3-epi-Eburnamenin-14α-ol (the name Eburnamin implies a 3β configuration) | 16-epi-Vobasan (Vobasan implies a 16S configuration) |
Racemates are named by citing the italicized prefix rac- (an abbreviation for racemo-) in front of the whole name of the compound including the prefix epi-, if present. The enantiomer for naming is chosen in accordance with RF-10.1.
RF-10.6. Relative Configuration
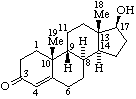
When the relative, but not the absolute, configurational relationships among asymmetric centers are known, the symbols R* and/or S* are used in accordance with Rule E-4.10 (ref 2). Alternatively, enantiomers of known relative, but unknown absolute configuration may be distinguished by a prefix (+)-rel- or (–)-rel-, where the plus and minus sign refer to the direction of rotation of polarized light at the sodium-D line. Hence, the dextrorotatory form of the following structure would be named: (+)-rel-17β-Hydroxy-8α,9β-androst-4-en-3-one (or enantiomer)
1. International Union of Pure and Applied Chemistry, "Nomenclature of Organic Chemistry. Section F: Natural Products and Related Compounds, Recommendations 1976", IUPAC Information Bulletin Appendices on Tentative Nomenclature, Symbols, Units, and Standards, No. 53, December, 1976. [also in: Eur. J. Biochem. 86, 1-8 (1978)].
2. International Union of Pure and Applied Chemistry, Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F and H, l979 edition, Pergamon Press, Oxford, 1979.
3. International Union of Pure and Applied Chemistry, A Guide to IUPAC Nomenclature of Organic Compounds, Blackwell Scientific Publications, Oxford, 1993. [Corrections see Pure Appl. Chem., 71, 1327-1330 (1999).]
4. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Joint Commission on Biochemical Nomenclature, "Nomenclature of Steroids", Pure Appl. Chem., 61, 1783-1822 (1989). [also in: Eur. J. Biochem., 186, 429-458 (1989) and pages xxx-lix in Dictionary of Steroids (Hill, R.A., Kirk, D.N., Makin, H.L.J. & Murphy, G.M., eds) Chapman & Hall, London 1991].
5. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Commission on Biochemical Nomenclature, "Nomenclature of Carotenoids", Pure Appl. Chem., 41, 405-431 (1975).
6. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Joint Commission on Biochemical Nomenclature, "Nomenclature of Vitamin D", Pure Appl. Chem., 54, 1511-1516, (1982). [also in: Arch. Biochem. Biophys., 218, 342-346 (1982), Endokrinol. Inf. No.2, 53-64 (1982), Eur. J. Biochem., 124, 223-227 (1982), and Mol. Cell. Biochem., 49, 177-181 (1982)].
7. International Union of Pure and Applied Chemistry, "Nomenclature of Fused and Bridged Fused Ring Systems", Pure Appl. Chem., 70, 143-216 (1998).
8. International Union of Pure and Applied Chemistry, "Nomenclature of Lignans and Neolignans (IUPAC Recommendations 2000)", Pure Appl. Chem., 72, 1493-1523 (2000).
9. International Union of Pure and Applied Chemistry and International Union of Biochemistry, Commission on Biochemical Nomenclature, "The Nomenclature of Steroids Revised tentative rules, 1967.", Pure Appl. Chem., 31, 285-322 (1972). This 2nd edition has been superceded by the 1989 edition (ref 4).