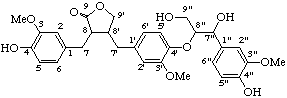Nomenclature of Lignans and Neolignans (Recommendations 2000)
LG-6 and LG-7
Trimers and Higher Analogues, Summary and References
Continued from LG-5 Stereochemistry
Contents
LG-6 Trimers of the C6C3 Unit and Higher Analogues
Higher analogues of the lignans and neolignans are composed of three or more C6C3 units. By analogy with the terpenoids these have been referred to as sesquineolignans, dineolignans etc. They may be named by an analogous way to the lignans and neolignans. The unprimed numbers are assigned to one of the terminal units and the other units are primed serially. Where there is a choice of locants preference is made in order to:
(a) less primed numbers
(b) position 8
(c) lower numbers
If there is an oxy linkage (see LG-1.4) this is quoted separate from and in front of the direct C-C linkages. If there is more than one oxylinkage then bis-, tris- etc is used. The number of C6C3 units is indicated by the appropriate prefix and neolignane:
Three C6C3 units sesquineolignane
Four C6C3 units dineolignane
Five C6C3 units sesterneolignane
LG-6.1 Sesquineolignans
Derivatives of the sesquineolignane skeleton are named following the recommendations of LG-2 to LG-5. If there is a choice the same criteria apply. In addition to unprimed numbers being preferred to primed, less primed numbers are preferred to ones with more primes.
Examples:
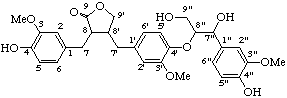
4,4",7",9"-tetrahydroxy-3,3',3"-trimethoxy-4',8"-oxy-8,8'-sesquineolignano-9,9'-lactone
(trivial name lappaol E)
(7"β,8β,8'α,8"α)-4,4",9"-trihydroxy-3,3",5'-trimethoxy-4',7"-epoxy-8,8':3',8"-sesquineolignano-9,9'-lactone
(trivial name lappaol A)
LG-6.2 Dineolignans
Dineolignans are named in a similar way to sesquineolignans. The locants for the additional C6C3 unit are triple primed.
Examples:
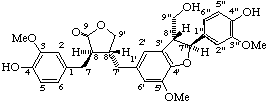
7,7'''-dihydroxy-3,3',3",4-tetramethoxy-3''',4'''-methylenedioxy-7',7"-epoxy-8,4':4",8'''-bisoxy-8',8"-dineolignane
(trivial name manassantin B)
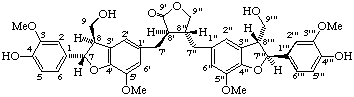
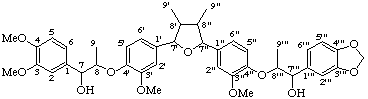
(7α,7'''β,8β,8'β,8"α,8'''α)-4,4''',9,9'''-tetrahydroxy-3,3''',5',5"-tetramethoxy-4',7:4",7'''-diepoxy-8,3':8',8":3",8'''-dineolignano-9',9"-lactone
(trivial name lappaol F)
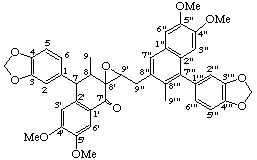
4',4",5',5"-tetramethoxy-3,4:3''',4'''-bis(methylenedioxy)-8',9'-epoxy-2',7:2",7'''-dicyclo-8,8':9',9":8",8'"-dineoligna-7",7'''-dien-7'-one
LG-7 Summary
To generate a semisystematic name for a lignan, neolignan, oxyneolignan or higher homologue the following stages should be applied in order:
(a) Identify the C6C3 units.
(b) Identify the preferred link(s) between the units with β,β' (LG-1.1) preferred to other carbon-carbon links (LG-1.2), and oxygen linked units (LG-1.4) least preferred. Homologues are considered similarly (LG-6).
(c) Identify parent lignane, neolignane, oxyneolignane or higher homologue, with indicated hydrogen (LG-1.3) if needed.
(d) Identify any additional rings present (LG-2.1), cleaved rings (LG-2.2), removed skeletal atoms (LG-2.3), added skeletal atoms (LG-2.4), replaced skeletal atoms (LG-2.5), bridges such as epoxy (LG-2.6) or rearranged skeletons (LG-2.7). In each case identify any indicated hydrogen if required (LG-1.3).
(e) Identify any side chain double bonds (LG-3.1).
(f) Identify, if present, the principal functional group named as a suffix (LG-4.1 to LG-4.5) with added hydrogen if appropriate (LG-4.4).
(g) Identify any further changes in hydrogenation level such as reduction of the aromatic ring (LG-3.2) or a double bond between an aromatic ring and side chain (LG-3.3).
(h) Identify any other functional groups (excluding the principal functional group) which are named as a prefix (LG-4.3 to LG-4.6).
(i) Orientate the structural formula to indicate stereochemistry (LG-5.1).
(j) Identify the stereochemistry present (LG-5.3 to LG-5.6) with appropriate configuration symbols (LG-5.2).
(k) Number the skeleton by application of the selection rules cited above applied in the same order until a unique numbering system is obtained (see also LG-1.5).
(l) Construct the name in the order of stereochemistry (j above), detachable prefixes in alphabetical order ignoring multiplicative prefixes (g and h above), non-detachable prefixes in order abeo- to cyclo- (i.e. reverse of order identified; d above), parent (c above), side chain double bonds (e above) and principal functional group (f above). Indicated or added hydrogen is included where appropriate.
References
1. Robinson, R. Proc. Univ. Durham Phil. Soc. 8, 14-59 (1927-8).
2. Haworth, R. D. Ann. Rep. Prog. Chem. 33, 266 (1936).
3. Hearon, W. M. and MacGregor, W. S. Chem. Rev. 55, 957-1068 (1955).
4. Freudenberg, K. and Weinges, K. Tetrahedron 15, 115 (1961).
5. Weinges, K., Nader, F. and Kunstler, K. (1978) Chemistry of Lignans ed. by C.B.S. Rao pp.1-37.
6. Gottlieb, O. R. Fortschr. Chem. Org. Naturstoff. 35, 1-72 (1978).
7. Whiting, D. A. Nat. Prod. Rep. 2, 191-211 (1985); 4, 499-525 (1987); 7, 349-364 (1990).
8. Dewick, P. M. and Jackson, D. E. Phytochem. 20, 2277-2280 (1981).
9. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC), Nomenclature of Organic Chemistry, Section F: Natural Products and Related Compounds, Recommendations 1976, Eur. J. Biochem. 86, 1-8 (1978); also pp. 491-511 in [ref 10] and pp. 19-26 in [ref 11].
10. International Union of Pure and Applied Chemistry Nomenclature of Organic Chemistry, Sections A, B, C, D, E, F, and H, Pergamon Press, Oxford (1979).
11. International Union of Biochemistry and Molecular Biology Biochemical Nomenclature and Related Documents, Second edition, Portland Press, London (1992).
12. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC), Nomenclature of Organic Chemistry, Revised Section F: Natural Products and Related Compounds, Recommendations 1999, Pure App. Chem. 71, 587-643 (1999).
13. International Union of Pure and Applied Chemistry A Guide to IUPAC Nomenclature of Organic Compounds, Recommendations 1993, Blackwell Scientific Publications, Oxford (1993); corrections Pure App. Chem. 71, 1327-1330 (1999).
14. IUPAC Commission on the Nomenclature of Organic Chemistry (CNOC), Nomenclature of Organic Chemistry, Section E: Stereochemistry, Recommendations 1974, Pure Appl. Chem. 45, 11-30 (1976) also on pp. 473-490 in [ref 10] and pp. 1-18 in [ref 11]. See [ref 15] for a more complete treatment of the R,S-system.
15. Cahn, R. S., Ingold, C. K. and Prelog, V. Angew. Chem. 78, 413-447 (1966); Angew. Chem. Int. Ed. Engl. 5, 385-415, 511 (1966); Prelog, V. and Helmchen, G., Angew. Chem. 94, 614-631 (1982); Angew. Chem. Int. Ed. Engl. 21, 567-583 (1982).
Return to Lignan home page.